Which Covalent Bond Is the Longest
Which bond is the longest. Single bonds are the longest of the three types of covalent bonds as interatomic attraction is greater in the two other types double and triple.

Solved A Covalent Bond Is The Longest A Bond In Which An Chegg Com
Which covalent bond is the strongest.

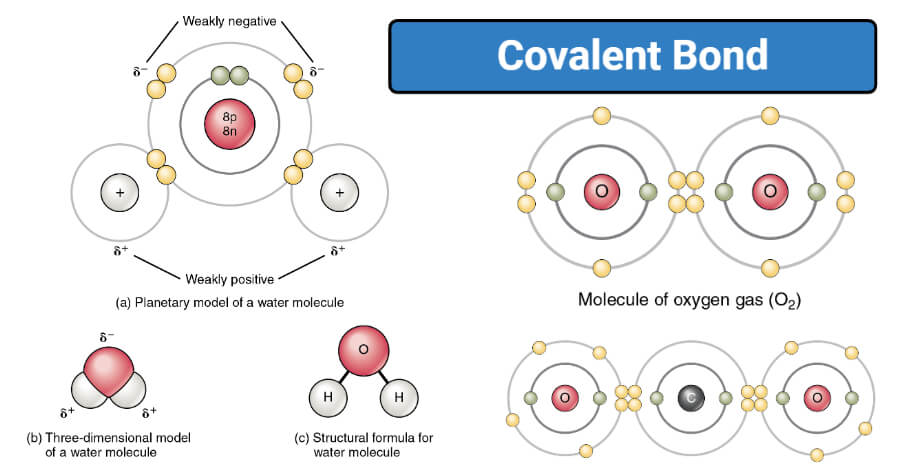
. A covalent bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. In covalent bonds such as those in methane and oxygen the valence electrons are shared between the atoms involved in the bond and they the electrons spend most of t. The order of bond lengths is single double triple.
A covalent bond is formed when valence electrons are shared between two atoms equally. Carbon-carbon bond C-C has the longest bond length. A double bond is a covalent bond in which _____ are shared between two atoms and are the longestshortershortest and strongeststrongerweakest type of covalent bond 2 pairs of electrons shorter stronger.
Generally the length of the bond between two atoms is approximately the sum of the covalent radii of the two atoms. Current record holder for the longest C-C bond with a length of 1862 pm is 18-Bis5-hydroxydibenzoadcycloheptatrien-5-ylnaphthalene one of many molecules within a category of hexaaryl ethanes which are derivatives based on hexaphenylethane skeleton. Better source needed For many molecules the sharing of electrons allows each.
C-C bond and C-H bond. Higher the number of bonds higher the nu. A covalent bond in which two pairs of electrons are shared between two atoms.
Its important to realize that in reality things arent more or less covalent. The weakest and longest covalent bond. Which covalent bond would be expected to be the strongest.
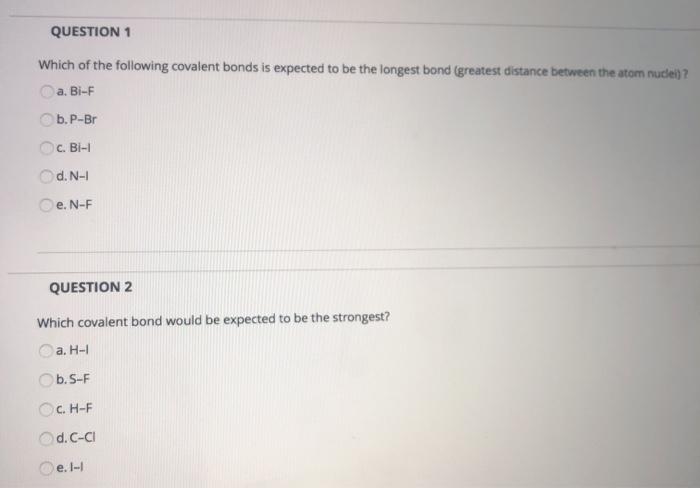
The longest covalent bond I can find is the bismuth-iodine single bond. S-F C-Cl H-F H-I I-I 3. The largest atoms should form the longest covalent bonds.
Generally the bond length of polar bonds C-O CN will be small compared to that of non-polar bonds ie. A covalent bond is a chemical bond in which pairs of electrons are shared between two atoms. The longest covalent bond I can find is the bismuth-iodine single bond.
So we look at atoms in the lower right corner of the Periodic Table. So we look at atoms in the lower right corner of the Periodic Table. A chemical bond formed when atoms share three pairs of electrons.
Sigma bond is considered to be the strongest bond where head to head overlapping occurs between atomic orbitals. C-C bond has the longest covalent bond distance. The reasoning for this is as follows.
The length of the bond is determined by the number of bonded electrons the bond order. Single bonds are the longest of the three types of covalent bonds as interatomic attraction is greater in the two other types double and triple. The order of bond lengths is single double triple.
What type of covalent bond is the longest. The higher the bond order the stronger the pull between the two atoms and the shorter the bond length. Answer 1 of 8.
Which of the five processes below absorb require energy to occur and which of the five release energy when they occur. What is the shortest covalent bond. All things are bonded due to the same electromagnetic properties arising out of the field equations in quantum mechanics whether those bonds are London dispersion Ionic covalent hydro.
The term covalent bond first came into use in 1939 although Irving Langmuir introduced the term covalence in 1919 to describe the number of electron pairs shared by neighboring atoms. They are also called molecular bonds. Know about covalent bonds in a detailed and interesting way subscribe BYJUS.
The increase in component bonds is the reason for this attraction increase as more electrons are shared between the bonded atoms Moore Stanitski and Jurs 343. But C-H bond length is greater than C-C bond length because the electronegativity difference is zero in C-C bond. Answer 1 of 4.
The define Bond-dissociation energy bond energy To correlate bond strength with bond length To define and used average bond energiesIn proposing his theory that octets can be completed by two atoms sharing electron pairs Lewis provided scientists with the first description of covalent bonding In this section we expand on this and describe some of the. Non polar bond length is higher than polar bond length. The largest atoms should form the longest covalent bonds.
Among C N O and H the covalent radius of C is maximum. Covalent bonds are formed by sharing of electrons. The increase in component bonds is the reason for this attraction increase as more electrons are shared between the bonded atoms Moore Stanitski and Jurs 343.
The increase in component bonds is the reason for this attraction increase as more electrons are shared between the bonded atoms Moore Stanitski and Jurs 343. Bond is located between carbons C1 and C2 as depicted in a picture below. Bond strength decrease in the following order covalent ionic metallic.
The shorter the covalent. 1 A SINGLE covalent bond between the same two atoms is the longest. Single bonds are the longest of the three types of covalent bonds as interatomic attraction is greater in the two other types double and triple.
Which of the following covalent bonds is expected to be the longest bond greatest distance between the atom nuclei.
Which Bond Is Longer C N Or C O Quora
Covalent Bond Definition Properties Types Examples
Solved Question 1 Which Of The Following Covalent Bonds Is Chegg Com
Comments
Post a Comment